Modern Physical Organic Chemistry
Eric V. Anslyn The University of Texas, Austin
Dennis A. Dougherty California Institute of Technology
ERRATA FROM THE FIRST PRINTING
(*) = New Figure enclosed here.
Page 34, Figure 1.9 (*)
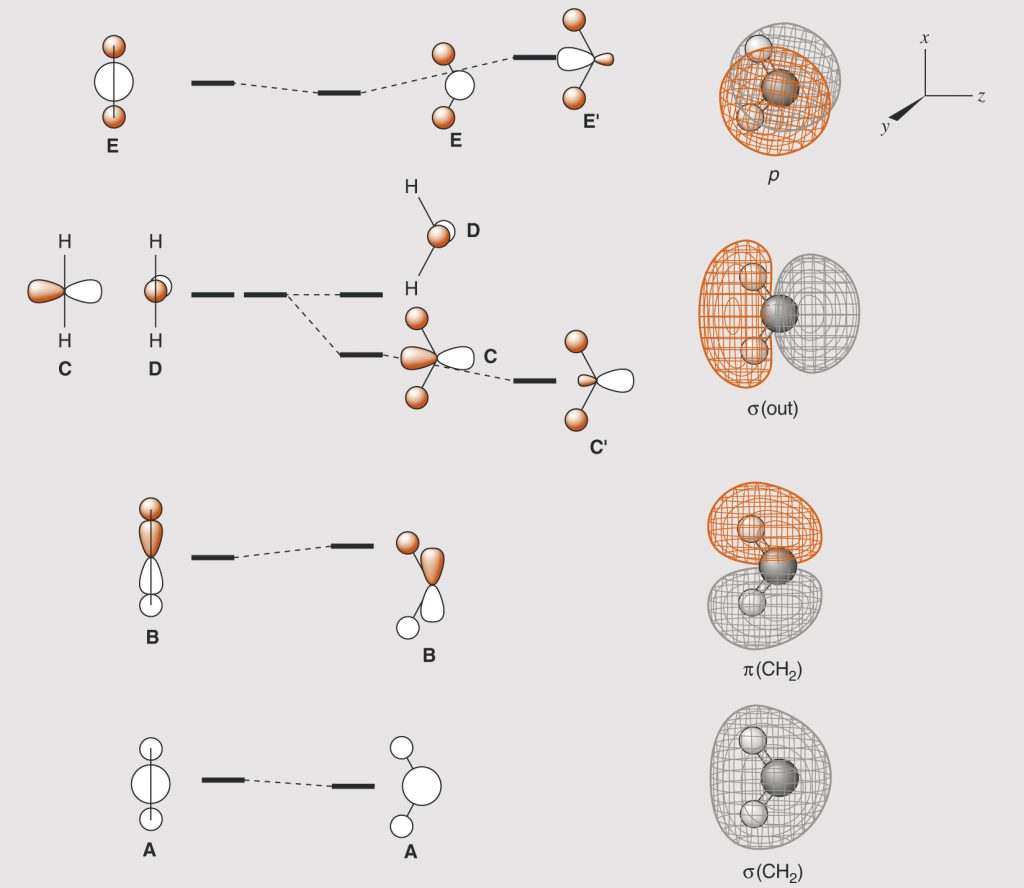
New figure with arrangement of the graphics in a slightly different manner.
Page 41, Figure 1.16 (*)
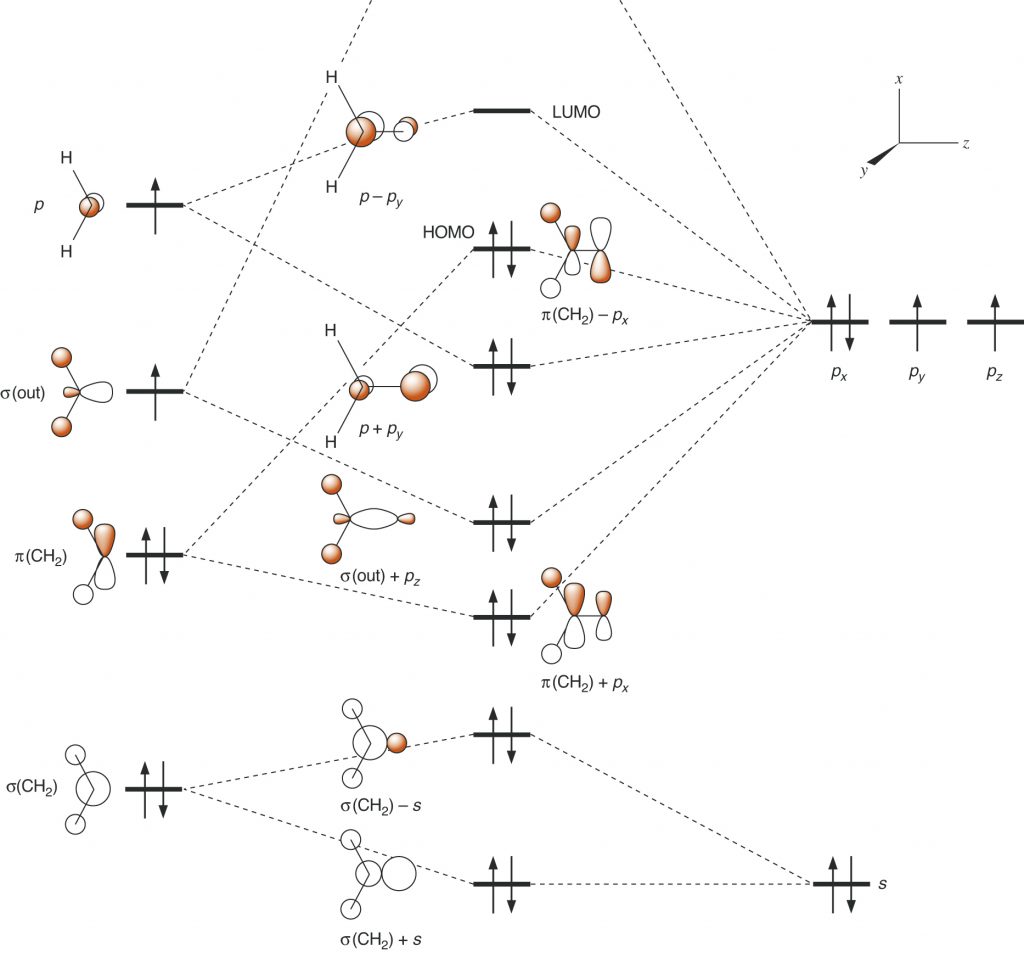
New figure with arrangement of the graphics in a slightly different manner. The phasing of a hydrogen atom in the third cartoon orbital is changed.
Page 42, Line 7
Replace “C-C” with “C-O”
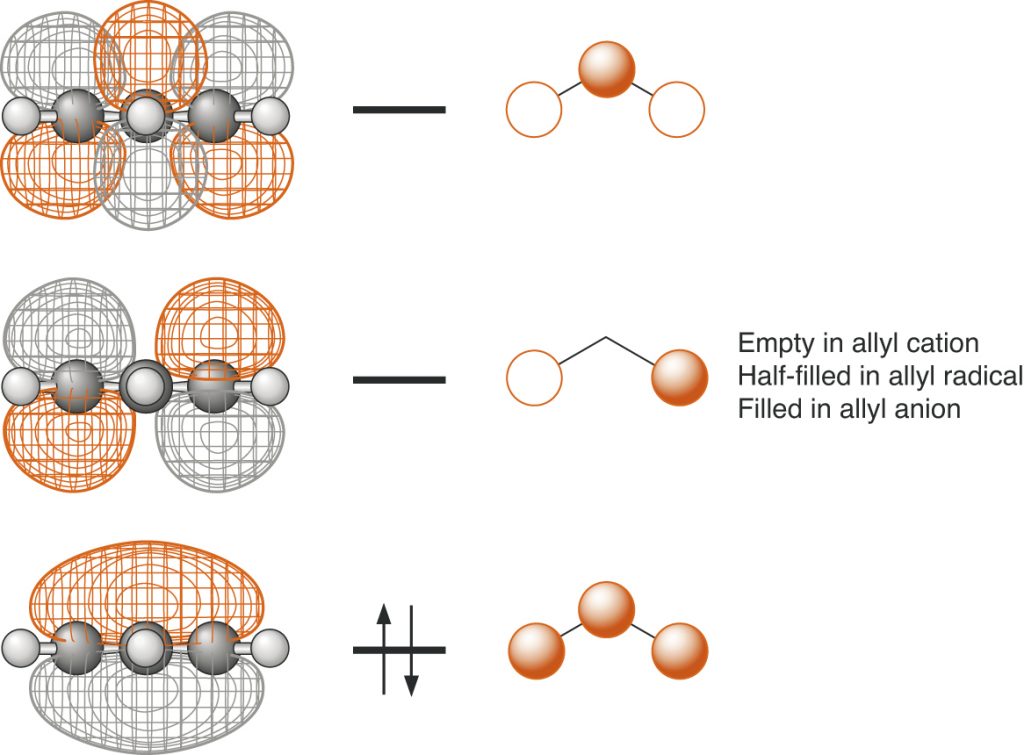
Page 49, Figure 1.22 (*)
New figure with phasing corrected.
Page 59
Line 7: Replace “A” with “i”
Line 17: Replace “C-C-C” with “N-C-N”
Page 79
Line 7L Replace “-29.43” with “-29.9”
Page 96
several changes in second paragraph:
Line 2: Replace -42.04 with –42.36
Line 6: Replace -42.04 with –42.36
Line 7: Replace –41.24 with –41.56
Line 9: Replace -42.04 with –42.36
Line 9: Replace –40.44 with –40.76
Page 110
Fifth full paragraph, line 3 replace “heptane” with “hexane”
Page 125
Fourth full paragraph, line 2 replace “stable” with “persistant”
Page 129
Fourth full paragraph, last line replace “Table 1.1” with “Table 1.4”
Page 141
Problem 34, second entry should be CH2CH3.
Page 148
Third line in parentheses, delete “=1”.
Page 283
Third paragraph, first line, replace “pKas” with “acidities”.
Page 290
Second and third sentences on the page to now read “The lower the energy of the orbitals containing the nucleophilic electrons, and the higher the energy of the empty orbital that can accept the electrons, the “harder” the respective base and acid. Lewis acids and bases with low energy empty and high energy filled orbitals are considered “soft”, respectively.
Page 312 (*)
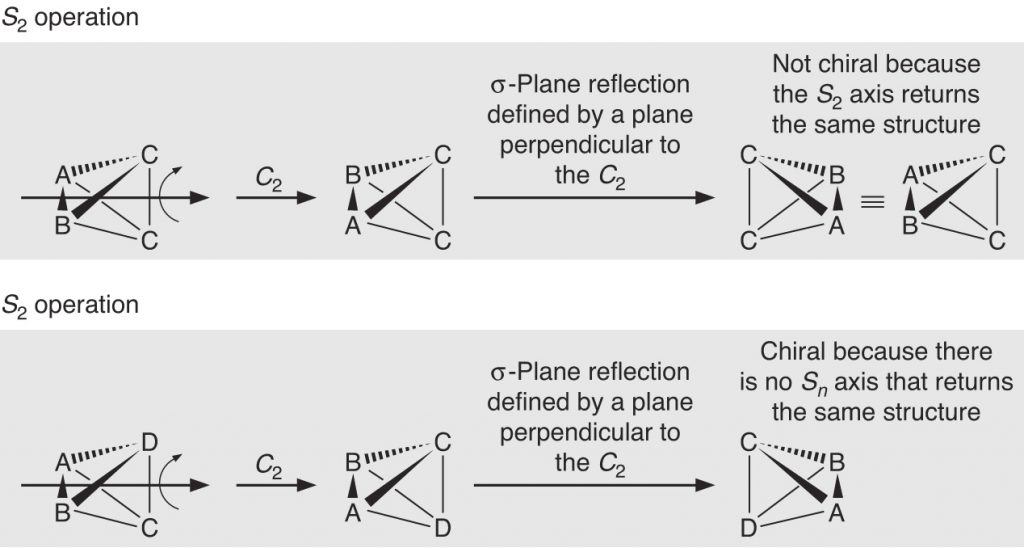
New graphical insert correcting a mistake in wedges and dashes.
Page 315
Fifth paragraph, seventh line insert “(n=1 in this case” at the end of the sentence containing “molecule” as the last word.
Page 361
First full paragraph, five lines from the bottom, delete “(R to I in each case)”.
Page 367
Second full paragraph, second line, replace “7.17” with “7.16”.
Page 387
In the Connections, the first chemical equation is missing a methyl group in the first radical product shown.
Page 392
Eq. 7.55, should = 0, while 7.56 should not = 0.
Page 426
Third full paragraph, second line, replace “Figure 8.4B” with “Figure 8.5B”.
Last line of the page, replace “Figure 8.4C” with “Figure 8.5C”.
Page 513
Line seven, replace “Figure 9.9 A” with “Figure 9.9 C”.
Page 528
There should be no “o” superscripts on the Gibbs activation energies.
Page 532
Structure of pyridoxal is wrong, There should be a methylene between the phosphate and the phenyl ring.
Page 707
Second paragraph, third line, replace “coordination” with “bonding”.
Page 727
First line of section 12.2.4 to now read “There is an entire class of eliminations that occur at metal centers where a group or atom from the ligand migrates to the metal while at the same time an unsaturated system is produced (Eq. 12.34).”
Page 748
Problem 9, the “Cp2” is missing on the “Ti” of the product.
Page 809
Eq. 14.6, there should be a “2” exponent on the “h-bar”.
Page 810
Fifth line from the bottom, insert “also called bra-ket,” in the parentheses before “Eq. 14.10”.
Page 831
Figure 14.9, There should be a prime on the DE going up.
Page 847
Figure 14.19, Delete the first sentence of the figure caption.
Page 851 (*)
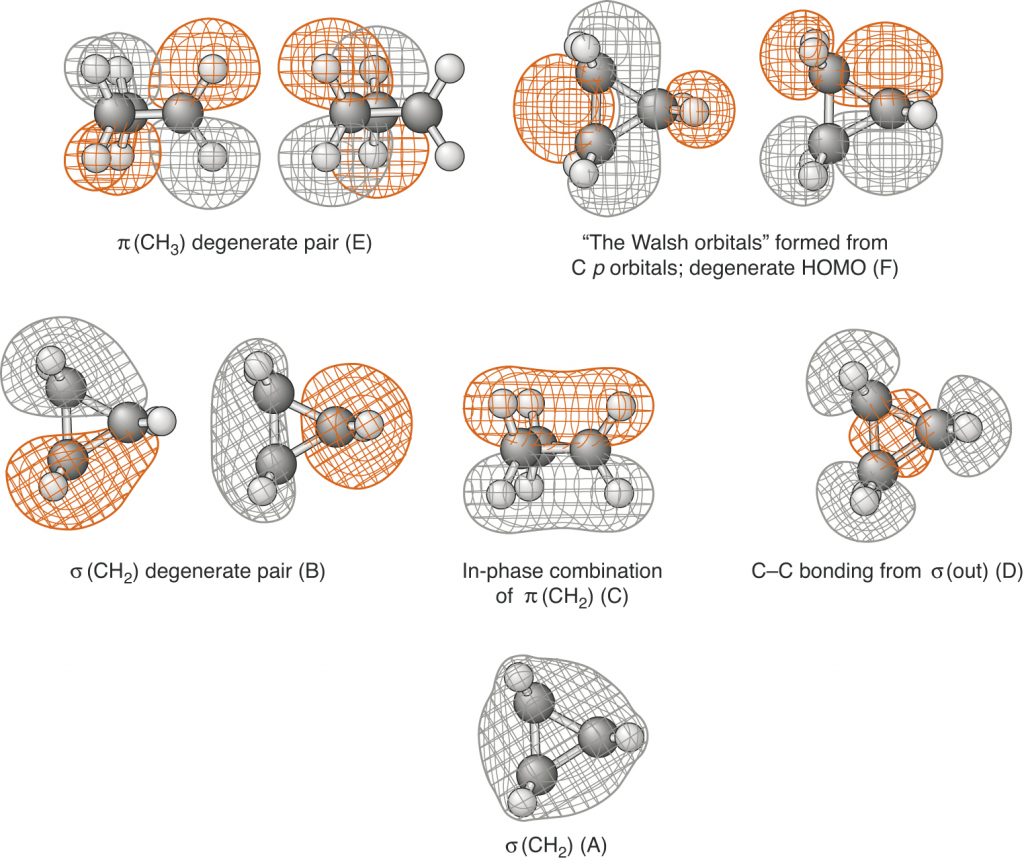
Figure 14.22 has been replaced with one that has the correct phasings.
Page 904 (*)
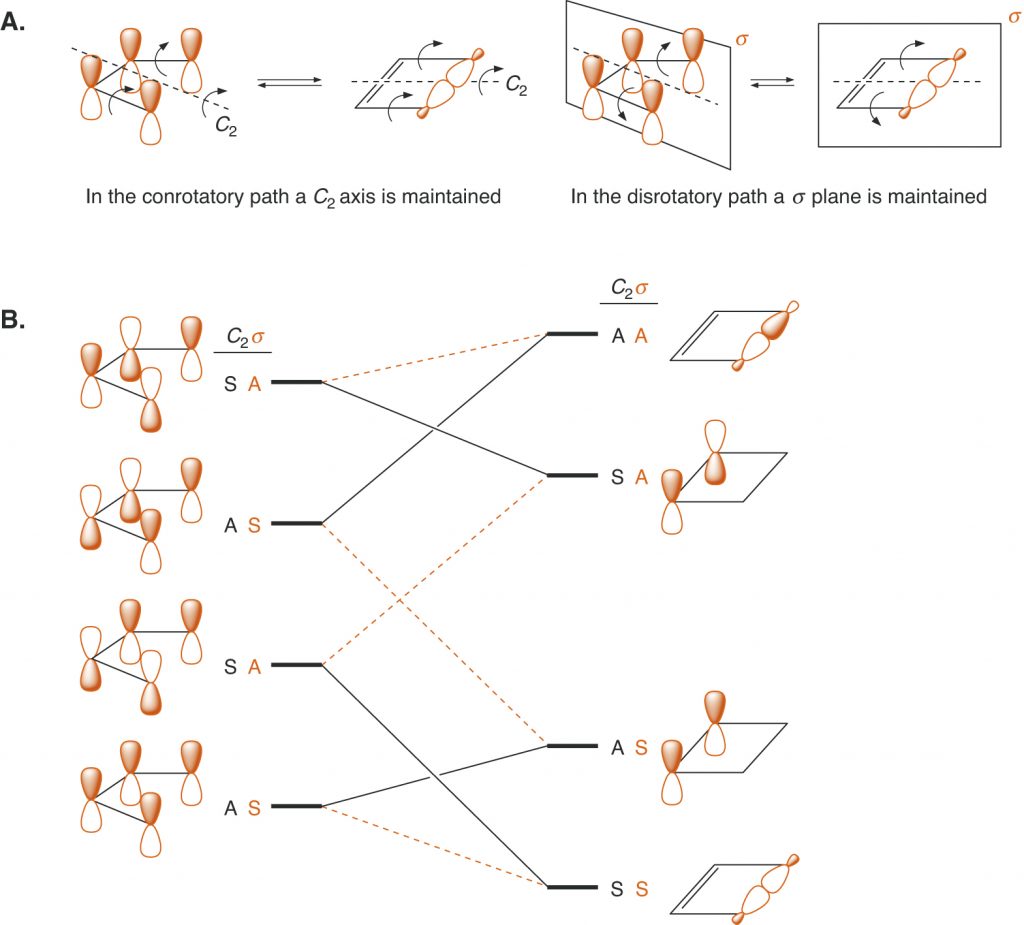
Figure 15.16, replaced with a figure that put new dashes in part A.
Page 1028
In Figure 17.19 part C, replace “m-xylene” with “m-Xylylene”.
Page 1074
Problem K, should have a second step – “2) NaH”.
Back Inside Cover
The caption starting “Substituent effects on the …” should be on the next page about the ESPs.
