General Chemistry
Fourth Edition
Donald A. McQuarrie, Peter A. Rock and Ethan B. Gallogly
Errata
Errata for the Fifth and Sixth printings
· Appendix I, Problem 25-30, answer should be 0.0545 V, not 0.0272 V.
Errata for the Fourth Printing
· Chapter 3, p. 96, in Figure 3.19, the boxes at the bottom of Groups 13 – 17 should be colored red, not green.
· Chapter 3, p. 103, Problem 3-44, the formula “K2CrO7” should be “K2Cr2O7“
· Chapter 26, p. 885, Example 23-11, the units in the denominator of the expression in the solution should be J*K-1*mol-1, not J-1*K*mol-1.
· Appendix I, Problem 13-60, answer should be 1.0×104 s-1.
Errata for the Third Printing
· Chapter 20, p. 762, Example 20-15, Solution (b) should read, “NH4F(s) forms an acidic solution…
· Chapter 24, Table 24.3: An error in the method of balancing oxidation-reduction reactions in base can result in an improperly balanced equation in cases where one of the species in the reaction equation contains hydrogen atoms but the other does not. For balancing in base a footnote to step 3 in the Table that reads:
*For metal hydroxides, directly add OH− ions. When neither side has an excess of oxygen atoms, balance any excess hydrogen atoms by adding a number of H2O molecules equal to the excess to the side deficient in hydrogen atoms and an equal number of OH− ions to the opposite side.
Skip Step 4.
· Appendix I, Problem 2-72, the correct answer for the % D in D2O is 1.3×10-6 %.
· Appendix I, Problem 10-64b answer should be 6, not 12.
· Appendix I, Problem 11-70 answer should be 9.24 kg.
· Appendix I, Problem 13-60, answer should be 1.0×104 s-1.
Errata for the Second Printing
· Chapter 1, p. 16, Example 1-7 solution: In the 2nd line of the mathematical solution the numerator “30×103 kJ” should be “30×103 J”.
· Chapter 6, p. 180, In text table should list Tl+ as “Thallium(I)” not “Thallium(II)”.
· Chapter 7, p. 214, Practice Problem 7-10, the formula of sodium carbonate should be given as “Na2CO3”.
· Chapter 7, p. 217, Example 7-11 solution: The first Lewis formula should not have a lone electron pair on the boron atom.
· Chapter 8, p 246, Figure 8.13: The bent molecular shape listed as AX2 should be listed as AX2E.
· Chapter 8, p 248, lower middle of page: The Lewis formula for SOCl2 should have two electron pairs on the sulfur atom and only two electron pairs on the oxygen atom, and so be written as:
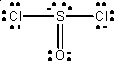
· Chapter 8, p 253, Trigonal planar figure at the bottom left of the page should be BF3, not BrF3, in drawing and label.
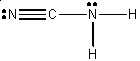
· Chapter 8, p 262, Problem 8-30, There is an extra hydrogen atom attached to the carbon atom. The correct Lewis formula is:
· Chapter 8, p 282, Practice Problem 9-4 solution: last sentence should read “…two Zn(sp) + Cl(3pz) sigma bonds of bond order 1.”
· Chapter 9, p 278, Example 9-3 solution: The bond orders should be 2.5, 3, and 2.5, respectively.
· Chapter 9, p300, Problem 9-8: the acetylide ion should be C22-, not C2+.
· Chapter 10, pp. 313-314, Practice Problems 10-1 and 10-3: The formula of (a) should be “NH4CH3COO(s)” in each problem (missing C in formula).
· Chapter 13, p. 425: The first sentence on the page should read, “100 000 pascals”, not “10 000 pascals” in two places.
· Chapter 13, p. 444: The second of Equation 13.17 should be “n2/(n1+n2)”, not “n2/(n1+n1)”.
· Chapter 14, p. 476, Example 14-2: The “30.0 grams of iron(III) oxide” given should be 60.0 grams.
· Chapter 14, p. 483, The first chemical equation on the page should read:
(1) C(s) + ½ O2(g) CO (g)
· Chapter 15, p. 533, Practice Problem 15-4: The structure labeled as “2-propanone (acetone)” should be labeled, “methanal (formaldehyde)”.
· Chapter 15, p. 550: The diagonal line on the figure in the margin should extend to the corners.
· Chapter 17, p. 622 (near the top): “(t = 2 t½ = 69 minutes)” should be “(t = 3 t½ = 69 minutes)”
· Chapter 17, p. 634 the last equation of the page should read: “rate of reaction = (0.50 M–1·s–1) [NO2]2”
· Chapter 18, pp. 661-662: N2O2 should be N2O4 on the two pages in many places (both in body of text and equations).
· Chapter 18, p. 678: Problem 18-30, the units of [S] in the data table should be [S]/10-2 mol·L-1, and those of R should be mmol·L-1·min-1.
· Chapter 18, p.681: Problem 18-64, the second equation should read, “rate of absorption = k1PA(1–θA)”
· Chapter 20, p 754: Section 20-10 Heading, “A (square) (aq)” should be “A–(aq)”
· Chapter 20, p763: two lines above the concentration table in the solution to example 20-16 should read, “Kb >> Kw” not “Ka >> Kw.”
· Chapter 21, p. 799: Practice Problem 21-8 should refer to Example 21-8, not Example 21-7.
· Chapter 22, p. 836: the third equation the page should read, “[Al3+] = …” not “[Ag3+] = …”
· Chapter 22, p. 842: the first chemical equation below equation 22.27 should have Cu2+(aq), not Cd2+(aq), as the product.
· Chapter 24, Table 24.3: An error in the method of balancing oxidation-reduction reactions in base can result in an improperly balanced equation in cases where one of the species in the reaction equation contains hydrogen atoms but the other does not. For balancing in base a footnote to step 3 in the Table that reads:*For metal hydroxides, directly add OH− ions. When neither side has an excess of oxygen atoms, balance any excess hydrogen atoms by adding a number of H2O molecules equal to the excess to the side deficient in hydrogen atoms and an equal number of OH− ions to the opposite side. Skip Step 4.
· Appendix I, Problem 13-60, answer should be 1.0×104 s-1.
· Replace APPENDIX I with this new pdf file.
